NDA MedTech Corporation
Leading design strategy for regulated MedTech products, transforming complex surgical data into safe, scalable, and human-centered experiences across software, hardware, and emerging platforms.
Tenure: June 2024 – Present
Domains: MedTech, Surgical Workflows, Regulated Systems
Platforms: Web, Mobile, Hardware Interfaces, Spatial (HoloLens)
Strategic Impact & Leadership
- Design Leadership in Regulated Environments: Own the end-to-end design strategy for surgical software and workflows under strict FDA and regulatory constraints, balancing usability, safety, and compliance across products.
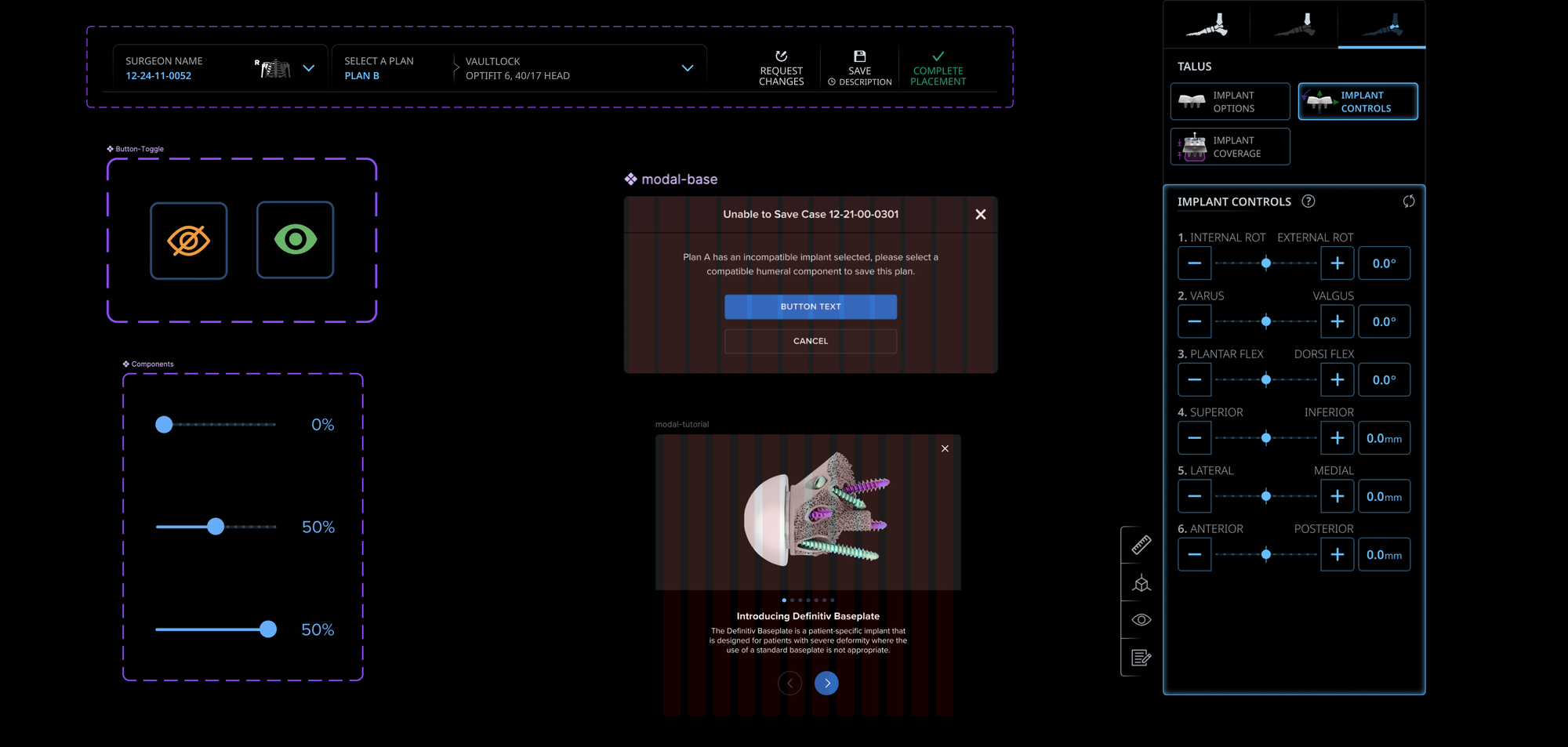
- Built a Scalable Health Design System:Designed and rolled out a comprehensive design system spanning mobile, web, and hardware interfaces — improving consistency, accelerating engineering delivery, and enabling long-term product scalability across teams.
- Making Critical Data Human-Readable: Transformed dense, high-pressure medical datasets into clear visual narratives that reduce cognitive load for surgeons — directly supporting faster comprehension and safer decision-making in clinical contexts.
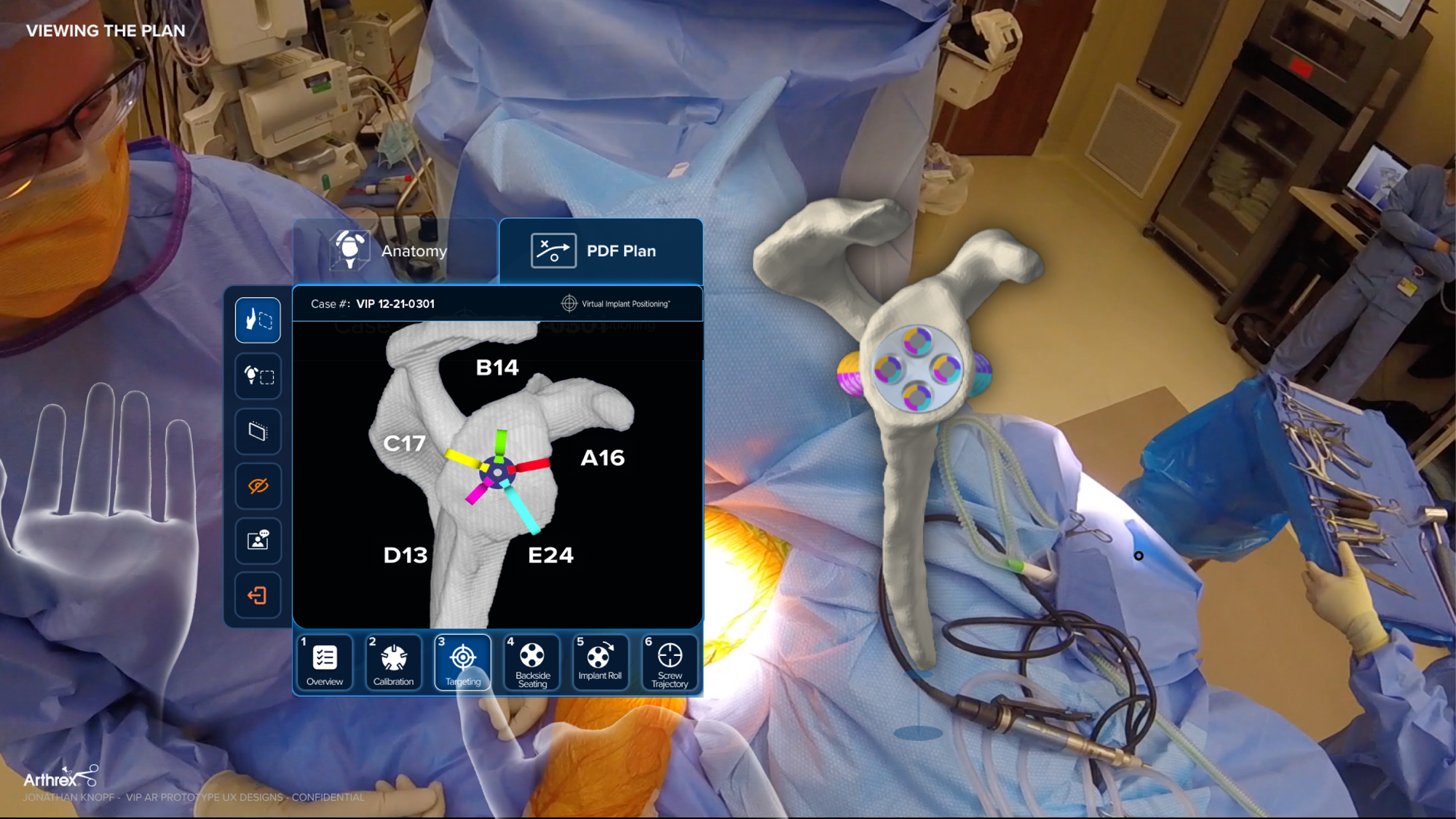
- Innovation Through Emerging Interfaces: Pioneered spatial interface concepts on Microsoft HoloLens to explore new ways of interacting with surgical data, using emerging technology to solve problems that traditional UI cannot.
Methods & Practice
- Design systems architecture for regulated products
- Complex data visualization for expert users
- Cross-platform interaction models (mobile, web, hardware, spatial)
- Design strategy under regulatory and technical constraints
- Stakeholder alignment across product, engineering, and leadership
- Prototyping and experimentation with emerging technologies
Tools
Figma, Miro/Lucid, design system frameworks, spatial interface tooling
Work examples: